China to supply COVID-19 vaccine to Pakistan as part of a trial agreement
As a part of a trial agreement, China has decided to supply...
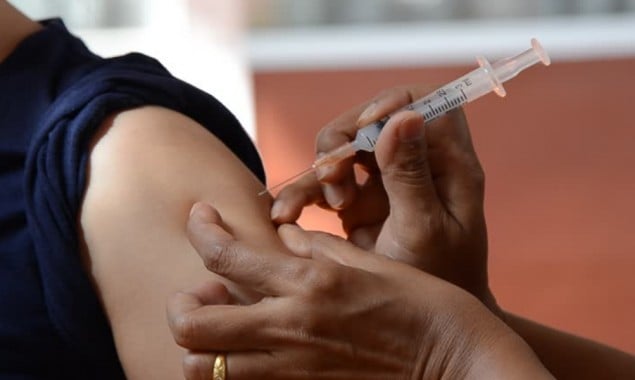
After the Drug Regulatory Authority of Pakistan (DRAP) approved the process on Monday, Pakistan has started the Phase-III of the clinical trial of coronavirus vaccine.
National Institute of Health (NIH) Islamabad spokesman said,
“This is the first time that the country is going through the phase-III clinical trial of any COVID-19 vaccine.”
He further added that,
“The Chinese company was already carrying out the clinical trial of the vaccine in Russia, Chile, Argentina, and Saudi Arabia.”
The coronavirus vaccine trial is being supervised by the NIH head, who also is the principal investigator of the program.
The Pakistan Health Research Council (PHRC) has also approved the clinical study that will be carried out in Karachi, Lahore, and Islamabad told the spokesman.
“Global clinical trials regulatory has also given nod to the process and it will be monitored in the country by the Global Scientific Committee.”
The spokesman said that the process will help to portray Pakistan’s image positive in the world.
It should be mentioned here that a Pakistani pharmaceutical company, AGM Pharma has signed an agreement with the Chinese Company to do the clinical trial of the COVID-19 vaccine at the NIH.
Catch all the Health News, Breaking News Event and Latest News Updates on The BOL News
Download The BOL News App to get the Daily News Update & Follow us on Google News.