Thai monks who test positive for drugs abandon the temple
According to local authorities, a small Buddhist temple in Thailand is now...
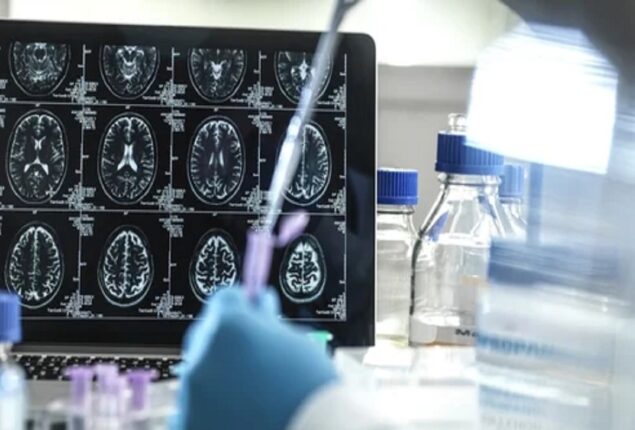
New Alzheimer’s drug slows memory loss
Doctors celebrate “new era” of medicine as study shows pill can delay Alzheimer’s symptoms.
Lecanemab eliminated aggregates of amyloid, a protein linked to the most common form of dementia, from patients’ brains.
The research, presented in San Francisco, sparked confidence among experts who had spent decades trying to understand the disease and discover a cure.
Rob Howard, professor of old age psychiatry at UCL, praised the results as “Wonderful and hopeful” – adding, “At last, we’ve made progress against this feared condition, and years of research have paid off.
“It’s monumental. This will boost hope that dementia can be healed.”
Top-line results were revealed in the fall, but many clinicians waited to celebrate until the Clinical Trials on Alzheimer’s Disease conference.
Lecanemab decreased memory loss and mental agility in moderate Alzheimer’s patients by 27%.
The medicine cleared so much amyloid protein that patients’ brain scans wouldn’t have shown enough Alzheimer’s disease to enter the trial.
The study reveals the medicine only works once brain amyloid levels are low.
After 12 months, treatment was useless, but after 18 months, it was effective.
Continued treatment should yield improved results, say, doctors.
Professor Nick Fox, director of the Dementia Research Centre at University College London, said: “It confirms a new age of disease modification for Alzheimer’s disease, after 20 years of hard work and many setbacks.”
Lecanemab doesn’t work. Slowing Alzheimer’s progression would be game-changing, delaying the need for professional care and giving them more time with their families.
Drug side effects exist.
One in eight lecanemab patients experienced brain edema and other abnormalities, likely due to amyloid removal. Most had brain scan-only issues. One in 30 had headaches or disorientation.
Some patients suffered a brain hemorrhage, but the treatment didn’t increase fatalities more than a placebo.
It highlights the need for meticulous therapy monitoring.
Fox prof: “Many of my patients would be willing to accept such a risk, nevertheless.
Lecanemab will be a huge problem for the NHS, and not just because it’s given every two weeks.
Most Alzheimer’s patients are diagnosed with intermediate symptoms, too late for lecanemab. Only 1% undergo a brain scan or spinal fluid biopsy to confirm their diagnosis.
Alzheimer’s Research UK’s Susan Kohlhaas: “The NHS isn’t ready for innovative dementia treatments.
Unless there are substantial changes in how people receive Alzheimer’s diagnostic testing, only 2% of eligible patients will get lecanemab.
Until now, only symptom-treating medications existed. If lecanemab is allowed for NHS use, delays in treatment will kill brain cells and advance the disease.
Prof John Hardy of the UK Dementia Research Institute in London called the drug “far overdue.”
Moreover, “I think it’s the end.
“We know the first step to developing successful medications. Future research could expand on this and lead to life-changing medicines for this condition.”
Catch all the Health News, Breaking News Event and Latest News Updates on The BOL News
Download The BOL News App to get the Daily News Update & Follow us on Google News.