Indian and Chinese forces battle with sticks and bricks
A deadly confrontation between Indian and Chinese forces was filmed. The film...
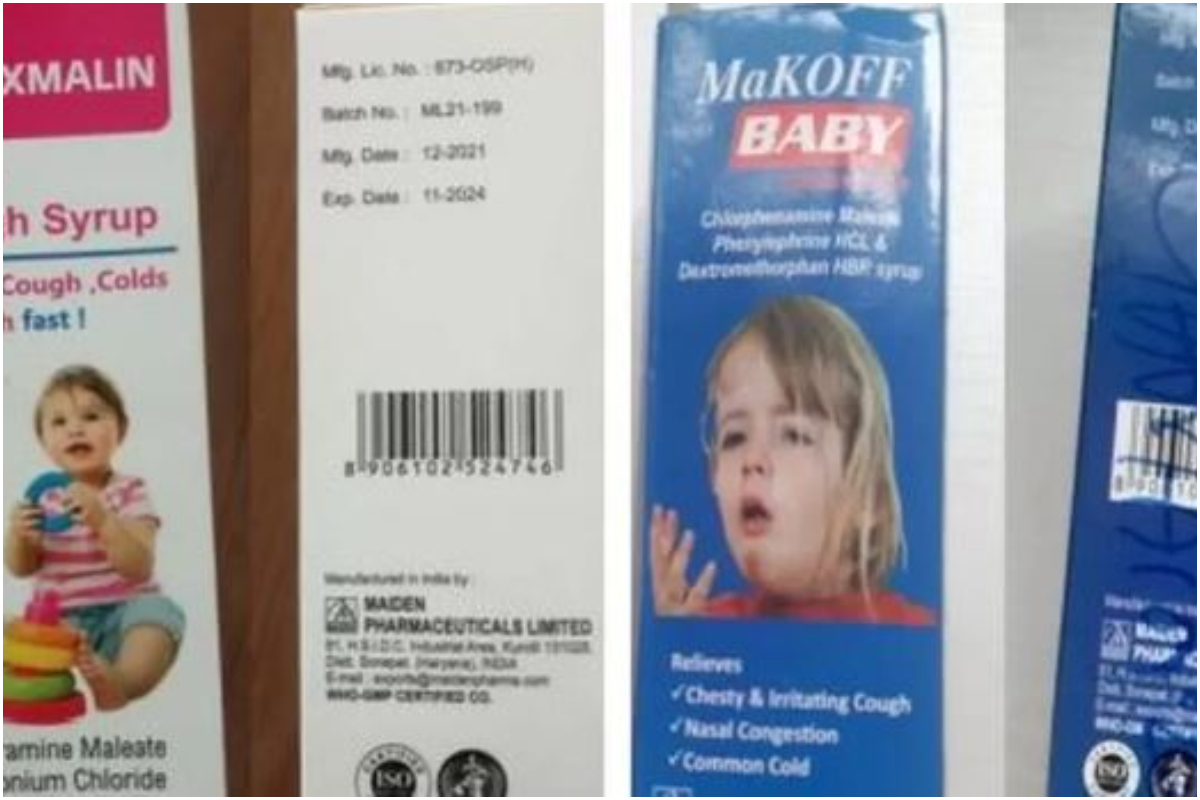
Maiden Pharmaceuticals: Gambia panel says Indian firm responsible for cough syrup deaths
According to India, tests conducted at home revealed that four cough syrups connected to infant deaths in the Gambia met safety requirements.
The four syrups, produced by India’s Maiden Pharmaceuticals, were cited by the WHO as having the potential to be responsible for at least 66 child fatalities in October.
But according to a letter from India’s pharmaceuticals authority, the WHO has not yet submitted any supporting documentation.
Africa receives several generic medications from India.
The letter, dated December 13 and addressed to Rogerio Gaspar, director of regulation and prequalification at the WHO, was written by Dr. VG Somani, India’s general drug controller. According to Reuters, the Indian health ministry provided reporters with a copy of the letter on Thursday.
In response to a WHO alert, India had stated in October that it was looking into the cough syrups.
The Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup samples that the WHO claimed to have examined were found to have “unacceptable quantities of diethylene glycol and ethylene glycol as contaminants.”
Humans are poisonous to diethylene glycol and ethylene glycol, which means ingestion could be lethal.
However, Dr. Somani claimed in his letter that the samples examined at a government lab “were found not to have been contaminated” with the substances. This week, the Regional Drug Testing Laboratory in Chandigarh’s government analyst “certified the cough syrup samples to be of standard quality,” according to Bhagwanth Khuba, the junior minister for chemicals and fertilisers.
A group of Indian experts are still reviewing the test results.
Dr. Somani noted that the WHO had been asked to provide the panel with “particular information” regarding “further elements required to prove the causality,” but that this information had not yet been provided. The information that the committee had requested was not made clear in the letter.
In the letter, it was also stated that the WHO’s October remark had been “sadly amplified by the global media,” harming the standing of Indian pharmaceuticals.
The WHO stepped in when The Gambia’s medical officials noticed a rise in acute kidney damage cases among children under the age of five in late July. Later, the government estimated that 69 kids had died as a result of these wounds.
The national regulator of The Gambia, however, stated in late October that it had not yet determined if the drugs were to blame for the fatalities.
“We still haven’t come to the conclusion that the medication is to blame. Several children died without getting any medication, “Tijan Fallow reportedly stated, according Reuters.
Catch all the Business News, Breaking News Event and Latest News Updates on The BOL News
Download The BOL News App to get the Daily News Update & Live News.