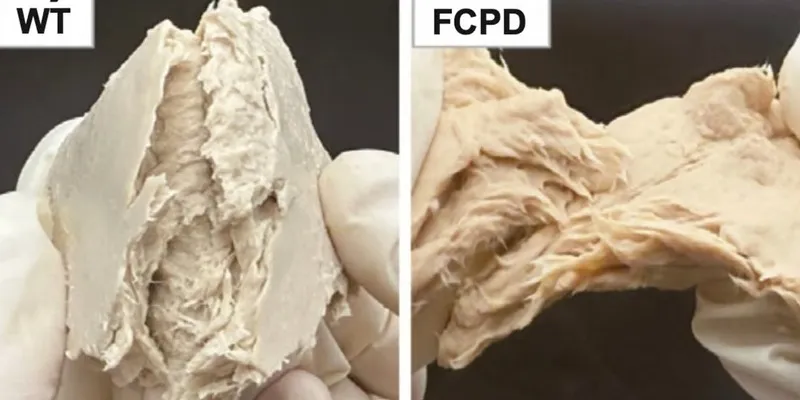
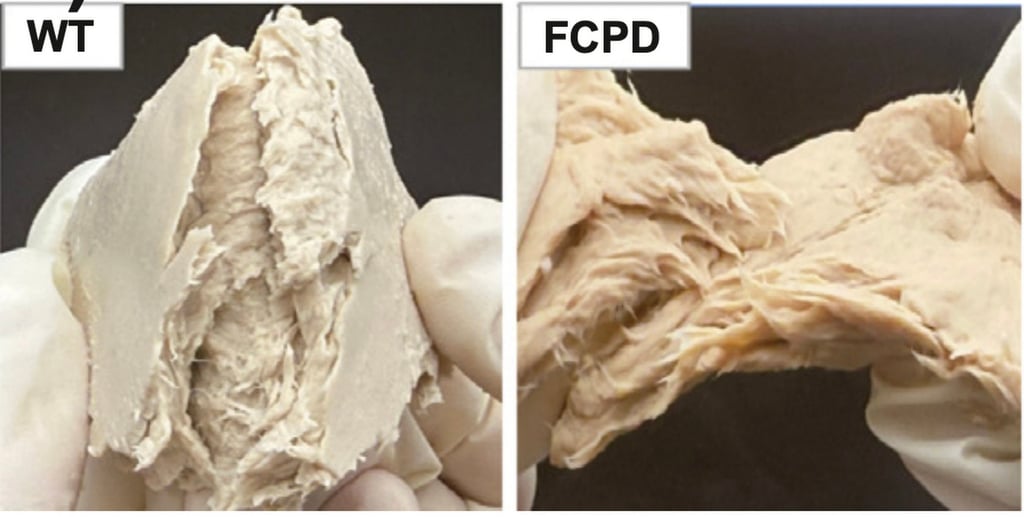
Scientists in China have genetically engineered a fungus that is widely used as a meat substitute, making it an even more sustainable and efficient source of protein than chicken, which is considered one of the lowest-impact farmed animals.
The research, conducted using CRISPR gene-editing tools, modified Fusarium venenatum without adding foreign DNA, according to a study published in Trends in Biotechnology.
Mycoprotein, often referred to as “fungus-derived protein,” is already renowned for its minimal environmental impact. It requires significantly less land, produces fewer greenhouse gases, and contributes less to water pollution compared to conventional animal proteins.
Moreover, its ability to replicate the texture and taste of meat has made it an increasingly popular alternative to traditional livestock farming.
However, concerns persist about the environmental costs of cell-cultured or lab-grown meat, with some studies suggesting that large-scale production might ultimately be more resource-intensive than even beef.
The groundbreaking research from China demonstrates substantial advancements in production efficiency. Compared to the unmodified variant, the engineered F. venenatum strain:
-
Consumes 44% fewer nutrients
-
Produces mycoprotein 88% faster
-
Reduces global warming potential by up to 61%, depending on the energy mix of the region
Furthermore, the modified strain requires 70% less land than the amount used for chicken farming in China, while also decreasing freshwater pollution risk by 78%, as reported by China Science Daily.
Liu Xiao, the lead researcher from Jiangnan University, stated: “These enhancements not only make the fungus more nutritious but also drastically lower its environmental footprint.”
Animal agriculture is responsible for nearly 40% of global agricultural land use and contributes to approximately 14.5% of global greenhouse gas emissions.
With growing concerns about climate change, water scarcity, and the urgent need for sustainable protein sources, scientists are increasingly turning to alternative solutions. While plant-based, microbial, and insect proteins are gaining popularity, lab-grown meat remains contentious.
A 2024 study from the University of California, Davis, found that large-scale cultured meat production could have a higher environmental impact than beef.
Nevertheless, mycoproteins derived from F. venenatum show considerable promise and have already been approved for consumption in the U.S., Europe, and Australia. Leading brands like Quorn have long utilized them to create meat-like products such as nuggets, fillets, and mince.
Traditional mycoprotein production requires large bioreactors and nutrient-dense media, a resource-intensive process with limited efficiency.
By employing CRISPR/Cas9, the researchers enhanced the fungus’s metabolic pathways, improving protein digestion, boosting nutrient quality, and minimizing overall nutrient requirements.
This innovation marks a significant leap toward more sustainable and efficient food production systems. The development of this genetically engineered fungus could play a pivotal role in shaping the future of alternative proteins, significantly reducing environmental impacts while meeting global food security needs.