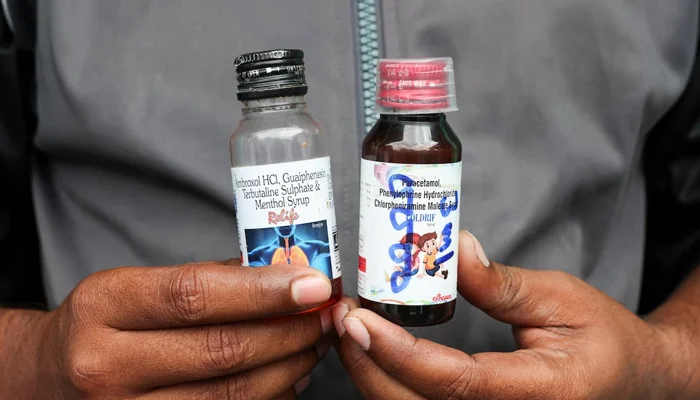
New Delhi: Indian authorities have launched an investigation after the deaths of 24 children linked to contaminated cough syrup, uncovering alarming safety lapses in the pharmaceutical supply chain.
According to an international news agency, health and drug safety officials in the southern state of Tamil Nadu suspect that Coldrif cough syrup may have been contaminated with the toxic chemical diethylene glycol (DEG) at the time its key ingredient, propylene glycol (PG), was supplied for production.
The report reveals that Sresan Pharmaceutical Manufacturer purchased the solvent on March 25 from local distributor Sunrise Biotech, which had sourced it the same day from a small firm, Jinkushal Aroma.
Both suppliers lacked the mandatory licences required for handling pharmaceutical-grade chemicals. In addition, Sunrise Biotech repackaged the PG without any protective seal—an unsafe practice that significantly increased the risk of contamination.
An investigation into Sresan’s manufacturing facility also exposed poor hygiene conditions, data falsification and several major regulatory violations. Officials stated that the factory had “hundreds of critical and major lapses,” although these were not directly linked to the children’s deaths. The company’s manufacturing licence has been cancelled, and its founder, G. Ranganathan, is currently in custody.
India’s pharmaceutical sector has faced criticism before, notably after similar incidents in 2022 and 2023 when contaminated Indian-made cough syrups caused child deaths in Africa and Central Asia.
Experts say DEG is sometimes either unintentionally or deliberately used as a cheaper substitute for PG, posing severe risks of kidney failure and potentially fatal outcomes, especially in children.
Meanwhile, the Indian government has stated that it is increasing inspections of drug manufacturing facilities and reviewing policies regarding the paediatric use of cough syrups.