Pfizer
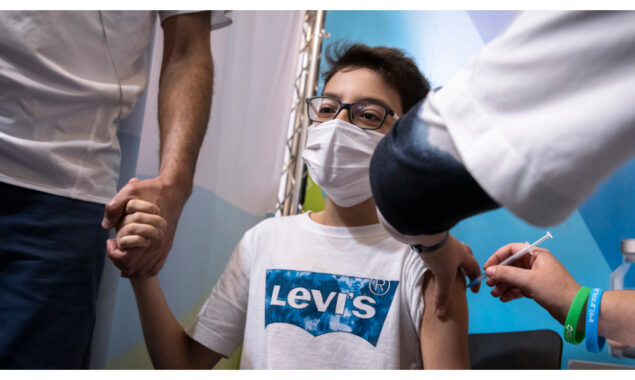
Pfizer and BioNTech announced positive from a scientific trial at the protection and immune reaction of a third dose of their Covid vaccine in kids elderly five through eleven, including they might soon seek regulatory authorization.on Thursday
Third doses of the vaccine are endorsed for those aged 12 and up, and a fourth dose become currently endorsed for people over 50.
Younger children — except for people with immune compromising conditions — have now not been eligible for the third, making them more liable to infection from Omicron and its BA.2 subvariant.
BA.2 is now the globally dominant strain, and is behind a current spike in cases in the northeastern United States.
In the phase 2/3 trial, the companies analyzed data from 140 children aged five through 11, approximately six months after the second dose.
The dosage in this group is 10 micrograms, which was selected for safety reasons as children are more susceptible to side effects. The dose for those 12 and up is 30 micrograms.
Across the 140 children analyzed, the third dose was well tolerated, revealing no new safety concerns.
They also analyzed blood sera from a group of 30 individuals, finding that a third dose caused a 36-fold increase in levels of infection-blocking neutralizing antibodies against Omicron, compared to two doses.
Pfizer and BioNTech plan to soon submit the data to the US Food and Drug Administration (FDA), the European Medicines Agency and other regulatory agencies.
Most countries, including the United States, haven’t yet authorized Covid vaccines for infants and very young children.
Last month, Moderna said it was pursuing acclaim for its vaccine in youngsters elderly six months through five years, using a -dose routine.
Pfizer’s vaccine for this group turned into supposed to be considered with the aid of the FDA in February however the organization postponed the meeting, as it wanted greater records on how it might perform with three doses.
Read More News On
Catch all the International News, Breaking News Event and Latest News Updates on The BOL News
Download The BOL News App to get the Daily News Update & Follow us on Google News.




