Indonesia: Merapi volcano erupts, spewing heated cloud
The Merapi volcano spewed a heated cloud up to seven kilometers high....
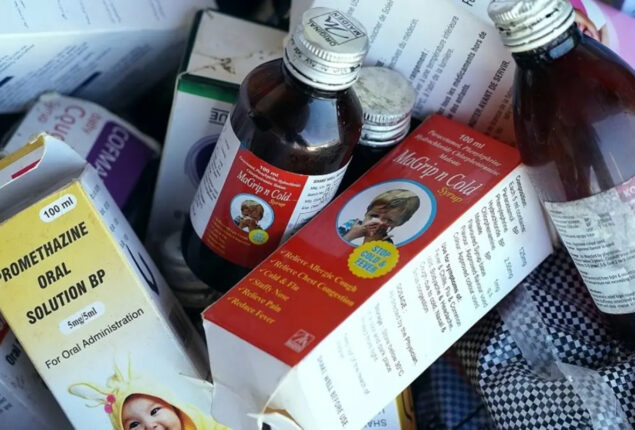
Indonesia cough syrup deaths: Court approves case, providing comfort to relatives
Once an Indonesian court approved a class action lawsuit, parents whose kids suffered fatalities or injuries from contaminated cough syrup sobbed in relief.
The fight of my child, who passed away last year at the age of four, was not in vain, said Nur Asiah.
The complaint was filed against the Indonesian government and eight pharmaceutical corporations by her family and the kin of 24 more victims.
Since 2022, acute renal damage has claimed the lives of more than 200 Indonesian youngsters.
Cough syrup contamination is not limited to Indonesia. In The Gambia and Uzbekistan, there have reportedly been about 100 fatalities.
While an investigation is ongoing in Indonesia, local officials claim that there is currently no proof connecting the instances to those in other nations. Six cough syrups produced in Indonesia and India have received alerts from the World Health Organization (WHO).
Before the court’s ruling, Nur Asiah told the news Indonesia, “I didn’t realize what I gave to my child was poison.
After getting a fever last year, her daughter Nasya was given a prescription for cough syrup. After taking the medication, she became severely ill and passed away three weeks later in the hospital.
The complaint wants $195,000 in damages for each child who is killed and nearly $130,000 for each youngster who is harmed. According to their attorney, additional parents will be permitted to join the lawsuit.
“Nothing can make up for what has happened, not even money. It won’t bring my child back “Nur Asiah stated while crying.
According to the lawyer for PT Afi Farma, whose cough syrup was used by the majority of the children in this instance, “it is not appropriate if the burden is just placed on the pharmaceutical business,” adding that the government should also be held liable.
For their brand of cough syrup, PT Universal Pharmaceutical Industries claimed to have used the same Indonesian Food and Drug Authority (FDA) certified system for almost 30 years and to have purchased the ingredients from suppliers who had received FDA approval.
The company’s attorney stated, “Honestly, pharmaceutical businesses are also victims – victims of a crime by the raw material suppliers.
A spokeswoman for the Health Ministry stated that a compensation plan was being developed.
We did our best to uncover the causes as rapidly as possible, exchange information with other nations and the WHO, and bring in antidotes to cure dangerous compounds.
Due to a global shortage of pharmaceutical-quality solvents, Indonesian authorities discovered that local chemical businesses used industrial-grade solvent material—Ethylene Glycol and Diethylene Glycol—in the syrup. The two ingredients are frequently found in antifreeze solutions for refrigerators and air conditioners.
Catch all the International News, Breaking News Event and Latest News Updates on The BOL News
Download The BOL News App to get the Daily News Update & Follow us on Google News.